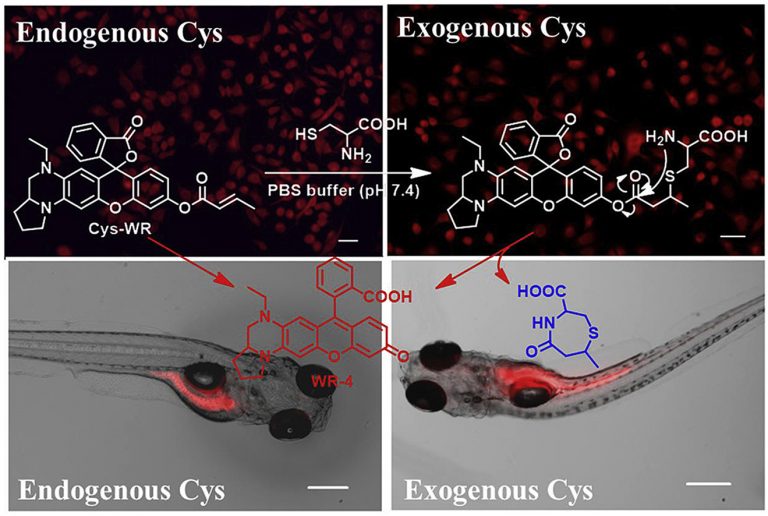
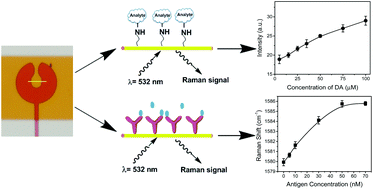
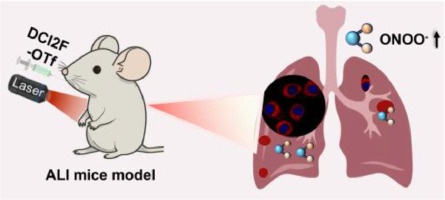
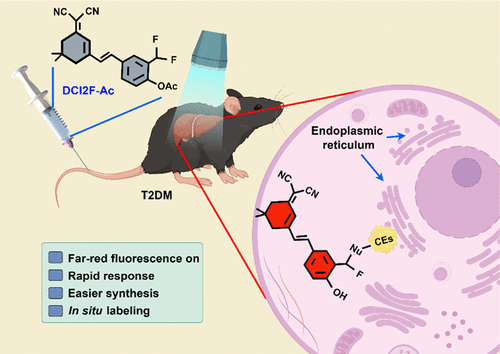
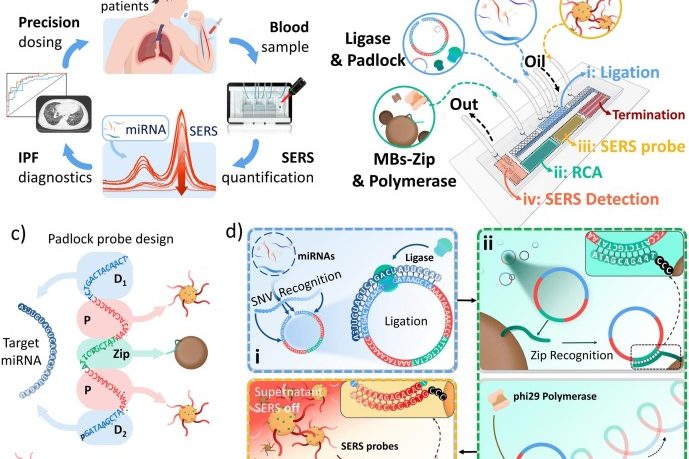
A microdroplet SERS-RCA biosensor with enhanced specificity and reproducibility for profiling dual miRNAs in idiopathic pulmonary fibrosis diagnosis and monitoring
A microdroplet SERS-RCA biosensor with enhanced specifi…
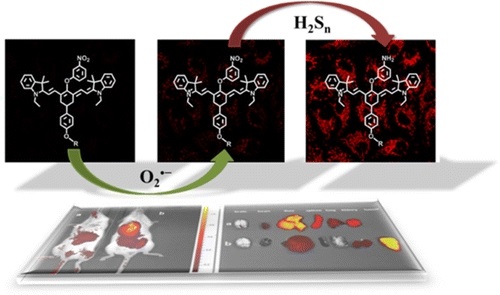
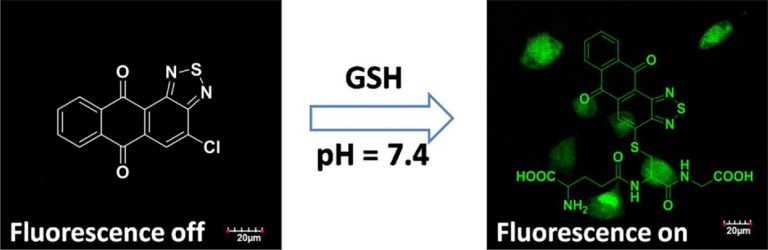
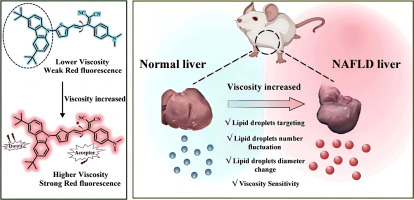
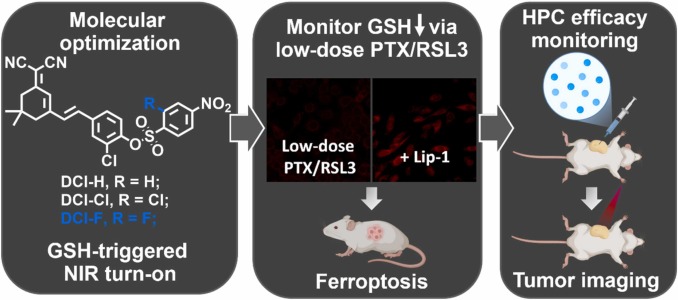
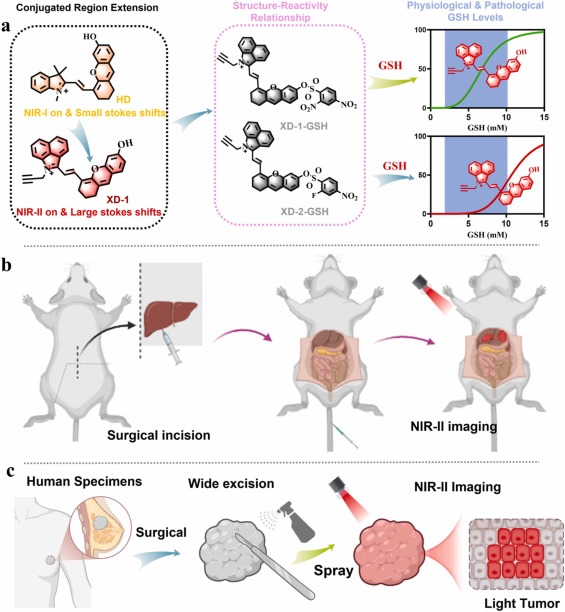
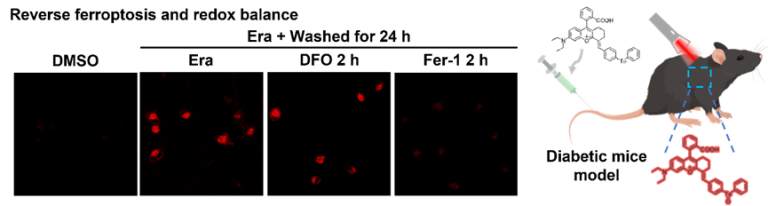
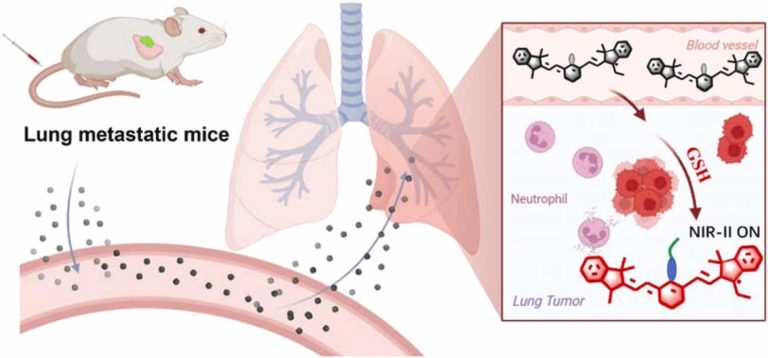
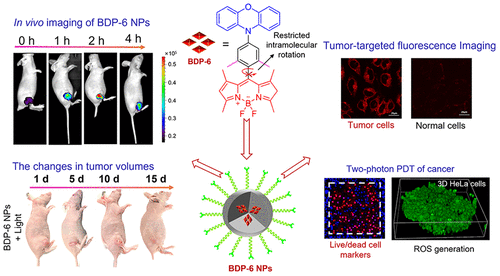
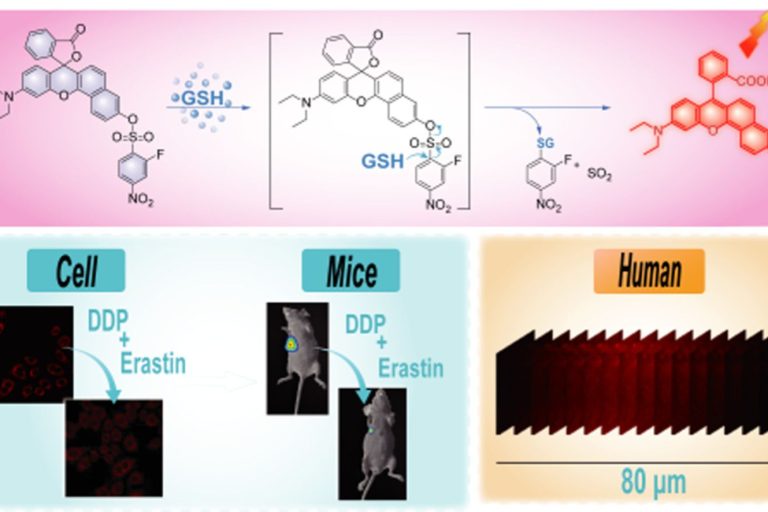
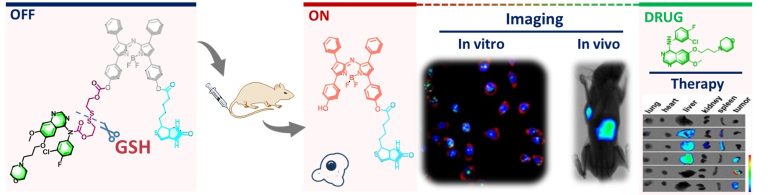
Evaluation of erastin synergized cisplatin anti-nasopharyngeal carcinoma effect with a glutathione-activated near-infrared fluorescent probe
Evaluation of erastin synergized cisplatin anti-nasopha…
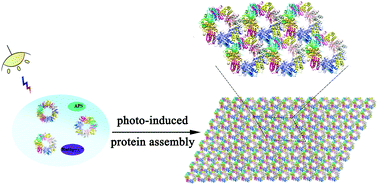
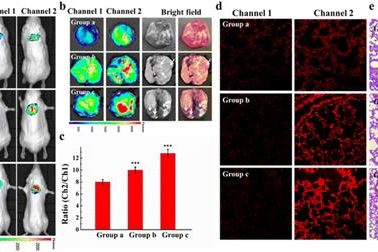
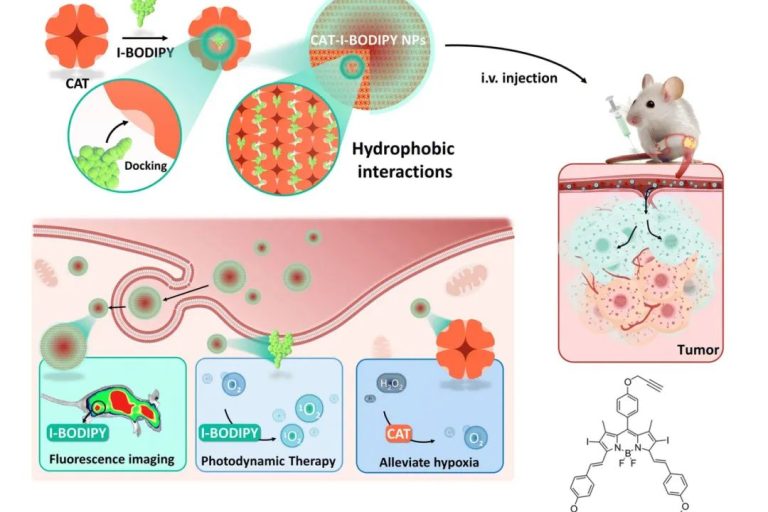
Multifunctional protein-based self-assembled nanoplatform: overcoming hypoxic tumor microenvironment for enhanced imaging-guided photodynamic therapy
Multifunctional protein-based self-assembled nanoplatfo…
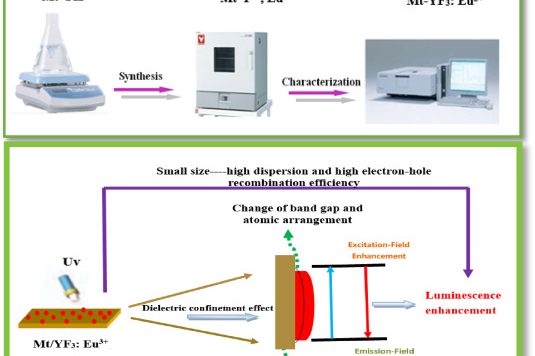
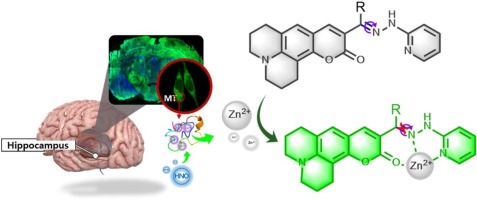
Synthesis of silicate clay minerals-based novel Mt/YF3:Eu3+ nanocomposites for regulated luminescent intensity-quantum yield-fluorescence lifetime
Synthesis of silicate clay minerals-based novel Mt/YF3:…
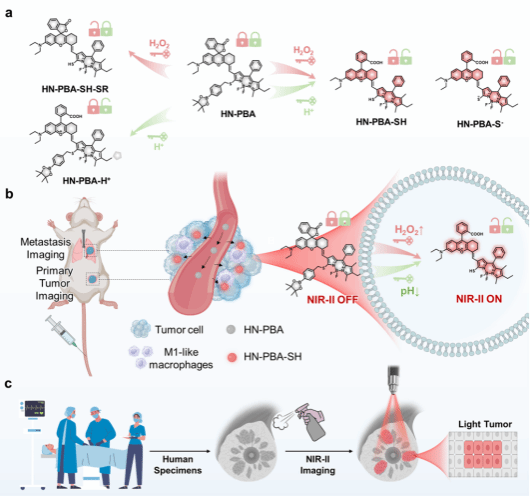
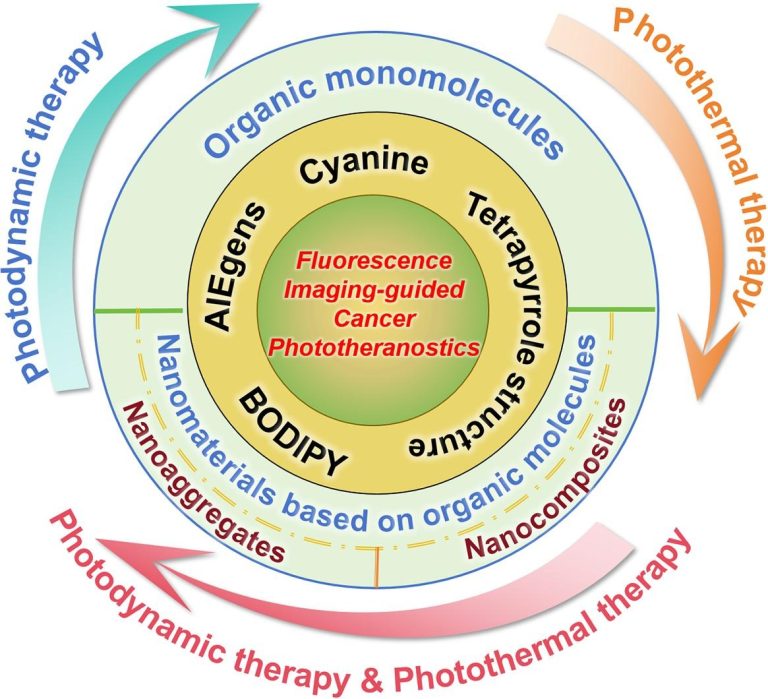
Fluorescence probes for lung carcinoma diagnosis and clinical application
Fluorescence probes for lung carcinoma diagnosis and cl…
Multifunctional protein-based self-assembled nanoplatform: overcoming hypoxic tumor microenvironment for enhanced imaging-guided photodynamic therapy
Biomater. Sci.|海南医学院于法标等:基于蛋白质的多功能自组装纳米平台—克服乏氧微环境用于增强成 …
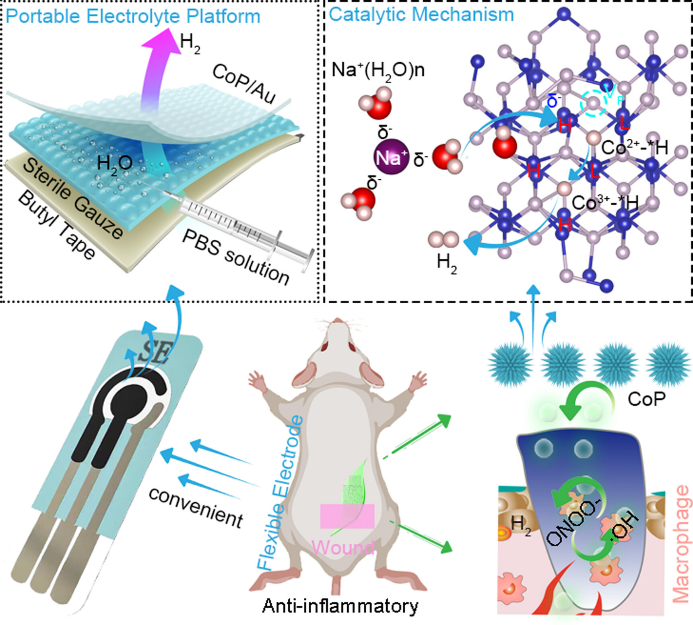
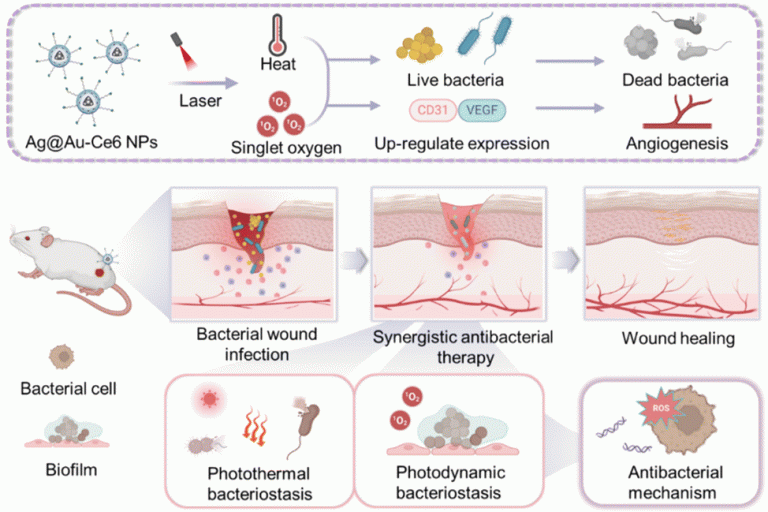
Hollow silver–gold alloy nanoparticles for enhanced photothermal/photodynamic synergetic therapy against bacterial infection and acceleration of wound healing
Biomater. Sci., 2023,11, 4874-4889.Full Text Shanshan L…
The effect of dark states on the intersystem crossing and thermally activated delayed fluorescence of naphthalimide-phenothiazine dyads
Beilstein J. Org. Chem. 2023, 19, 1028–1046.Full Text L…
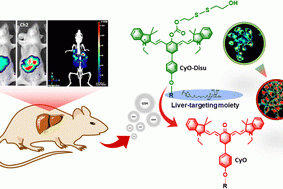
Bioimaging of glutathione variation for early diagnosis of hepatocellular carcinoma using a liver-targeting ratiometric near-infrared fluorescent probe
J. Mater. Chem. B, 2023, 11, 6612-6620.Full Text Xiaoyu…
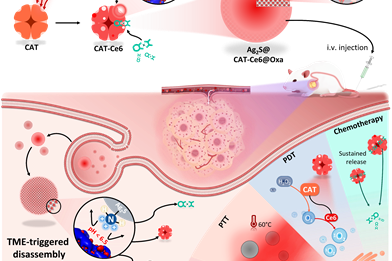
Controllable Regulation of Ag2S Quantum-Dot-Mediated Protein Nanoassemblies for Imaging-Guided Synergistic PDT/PTT/Chemotherapy against Hypoxic Tumor
Advanced Healthcare Materials,2023-06-12Full Text Mengj…
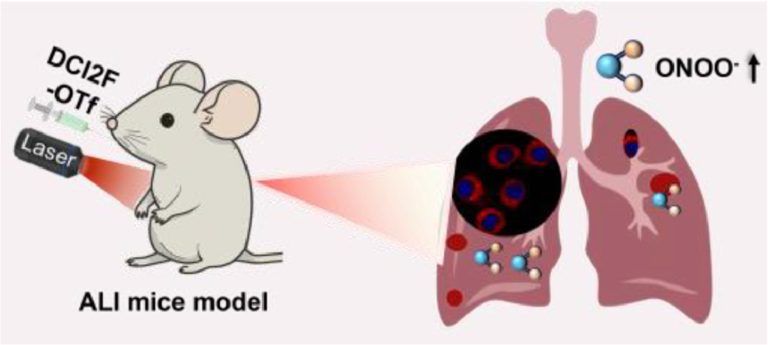
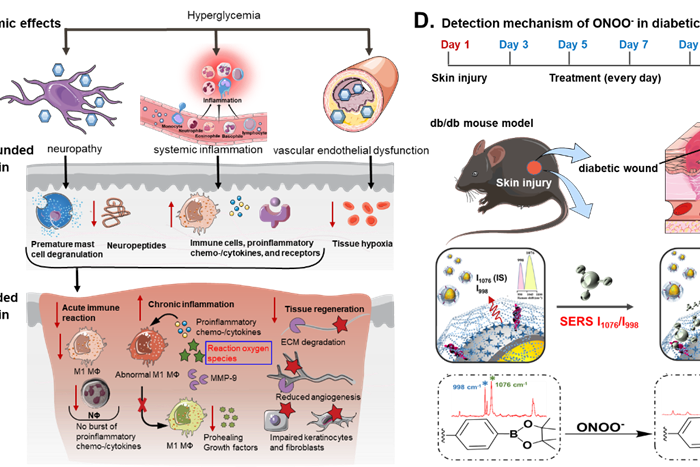
Ratiometric SERS imaging for indication of peroxynitrite fluctuations in diabetic wound healing process
Received 27 March 2023, Revised 13 May 2023, Accepted 7…
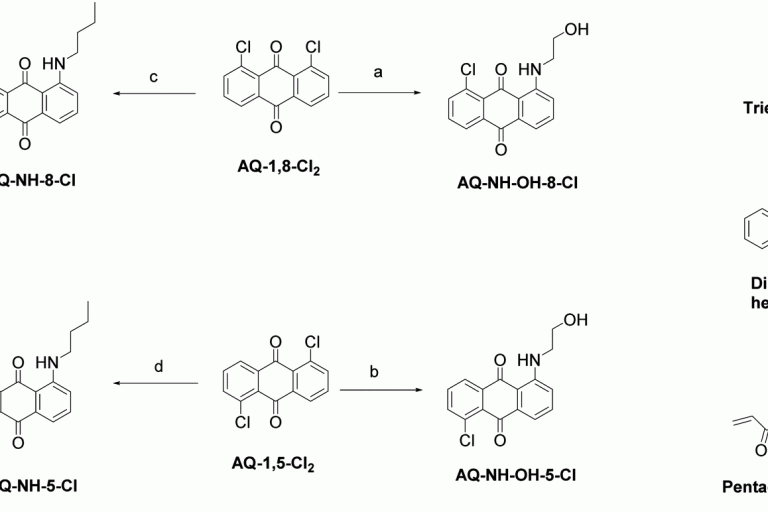
Preparation of amino-substituted anthraquinone: study of the intersystem crossing and application as efficient photoinitiators for photopolymerization
Received 21st March 2023 , Accepted 28th April 2023 Fi…
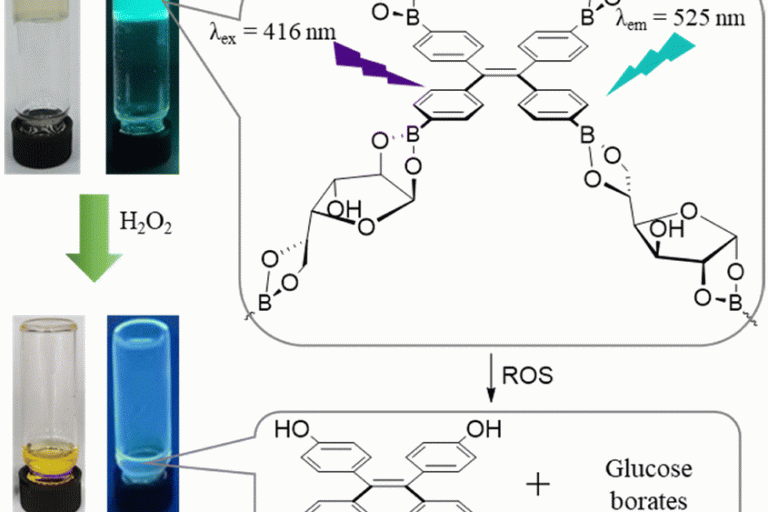
Macroscopic and fluorescence detection of reactive oxygen species by using a glucose-linked tetraphenylethylene polymer gel
Received 24th February 2023 , Accepted 10th April 2023F…
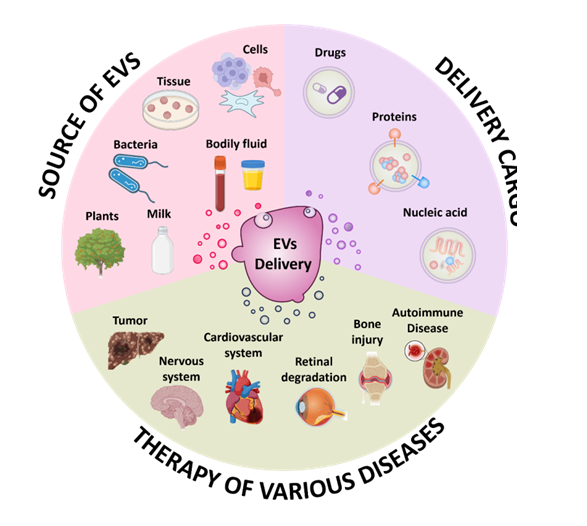
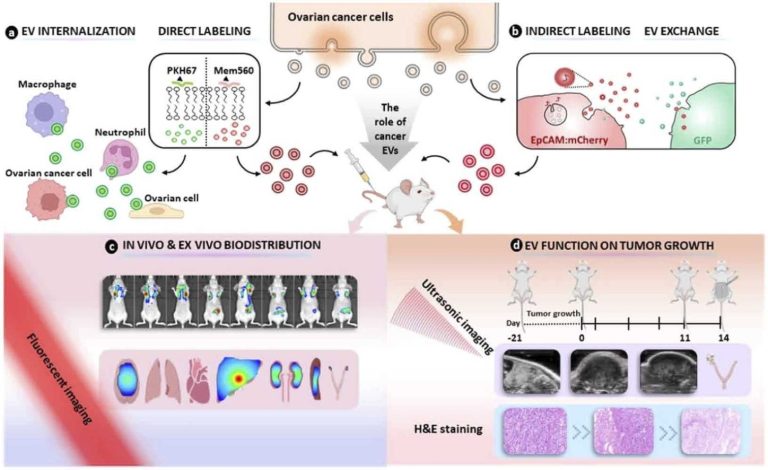
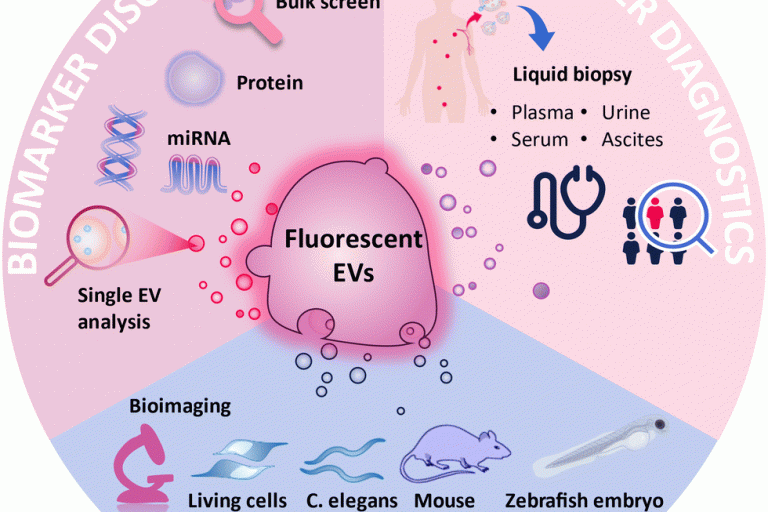
荧光标记的细胞外囊泡在体内和体外的不同生物应用Fluorescence labeling of extracellular vesicles for diverse bio-applications in vitro and in vivo
This article is part of the themed collection: 2023 Pio…
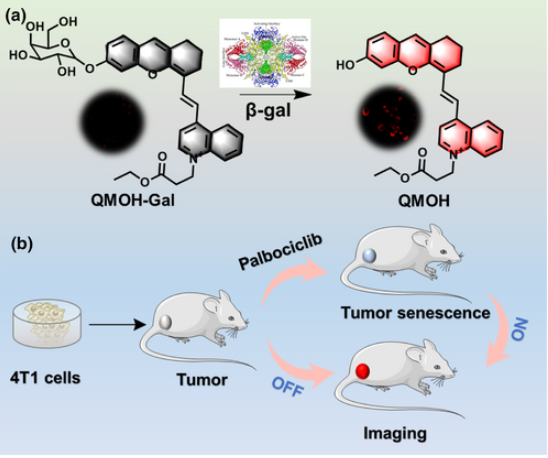
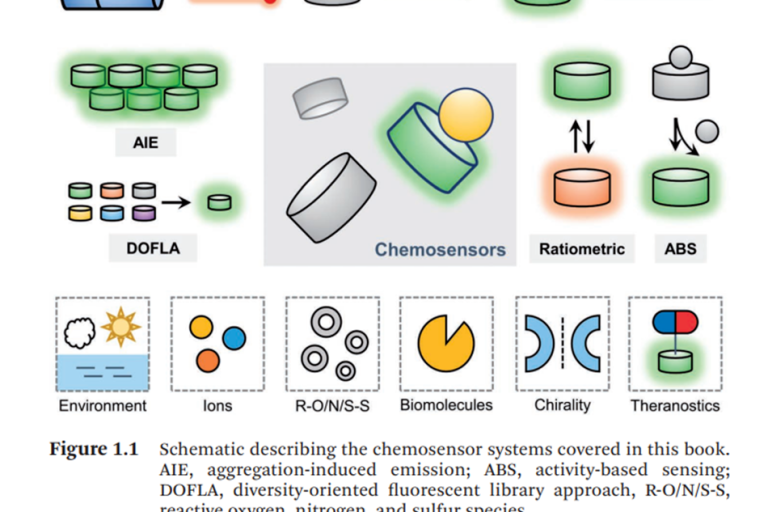
新书推荐 Fluorescent Chemosensors 《荧光化学传感器》Chapter 13 用于硫烷硫和活性硒检测、成像的分子荧光探针
Fluorescent Chemosensors Edited byLuling WuAdam C Sedgw…
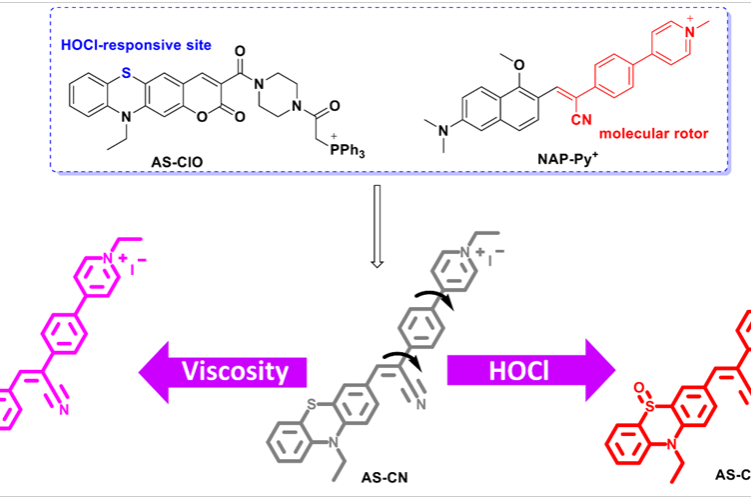
A two-pronged detection of atherosclerosis with a dual-channel fluorescent probe for viscosity and hypochlorous acid
Chemical Engineering Journal , 2023 Full Text Zhenkai …
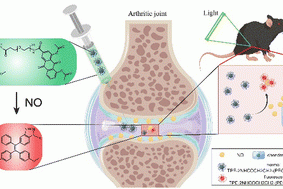
A nitric oxide responsive AIE probe for detecting the progression of osteoarthritis
Journal of Materials Chemistry B , 2023 Pan Jin, Guoji…
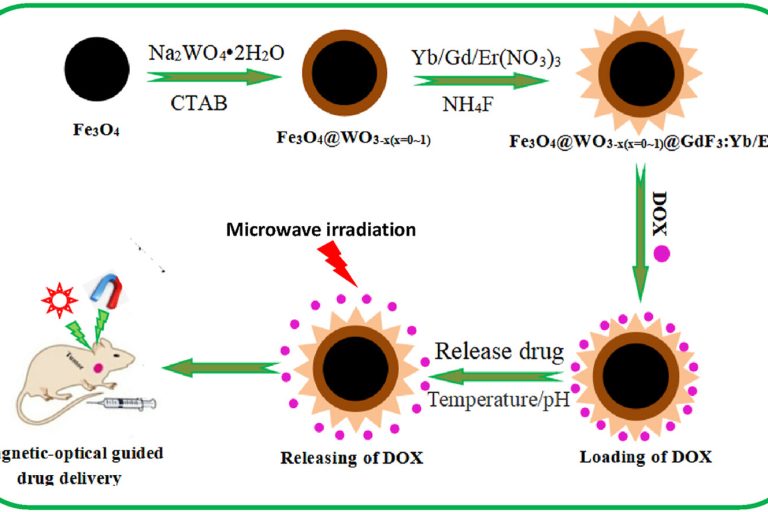
A novel multifunctional carrier with magnetic-NIR luminescent-microwave heating characteristics for drug delivery
Journal of Drug Delivery Science and Technology , 2023,…
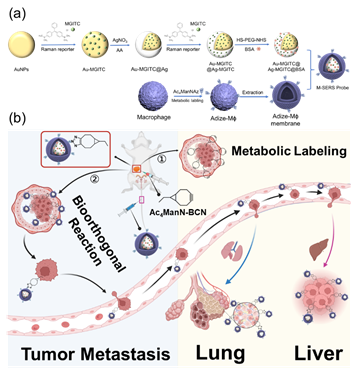
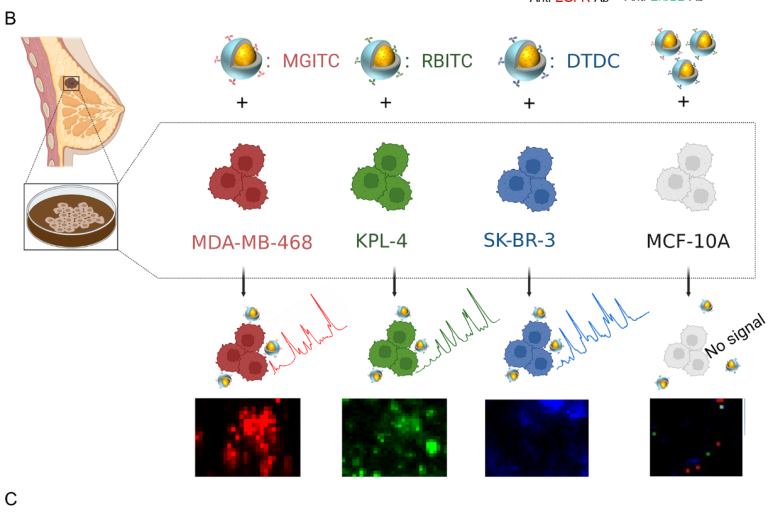
Highly sensitive surface-enhanced Raman scattering (SERS) imaging for phenotypic diagnosis and therapeutic evaluation of breast cancer
Chemical Engineering Journal , 2023, 1415025,Text Full …
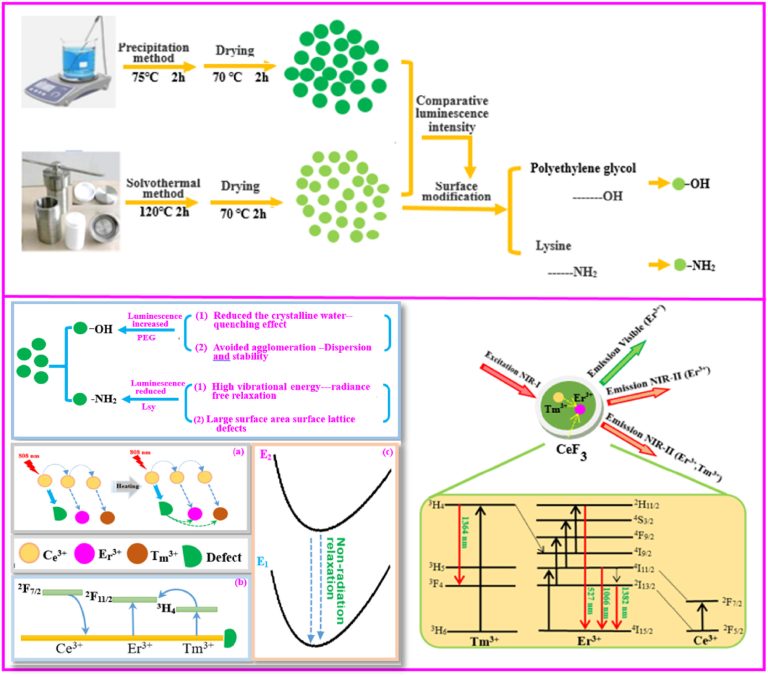
Synthesis of novel halloysite@YF3:Ce3+,Tb3+ nanocomposite for enhanced luminescent properties
Advanced Powder Technology , August 2022, 103775,Txet F…
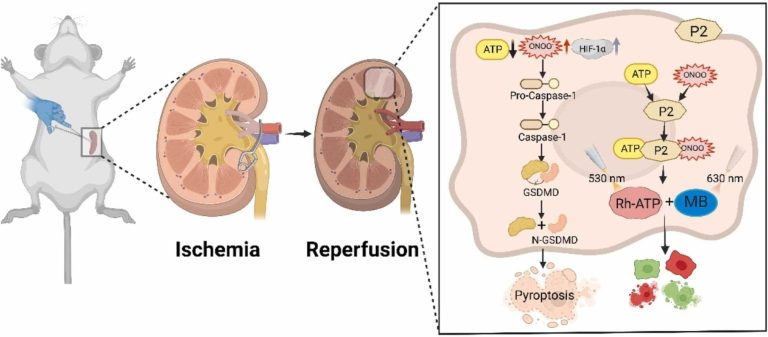
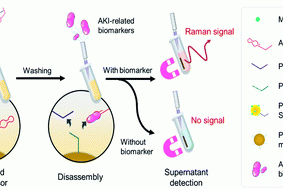
SERS based Y-shaped aptasensor for early diagnosis of acute kidney injur
RSC Advances, 2022, 12, 15910 – 15917 ,Text Full …
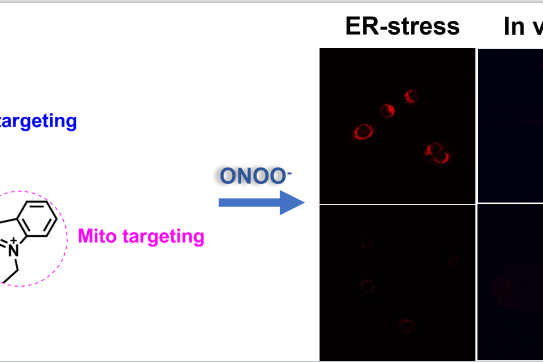
Construction of a mitochondria-endoplasmic reticulum dual-targeted red-emitting fluorescent probe for imaging peroxynitrite in living cells and zebrafish
Chemistry – An Asian Journal , May 2022, e2022003…
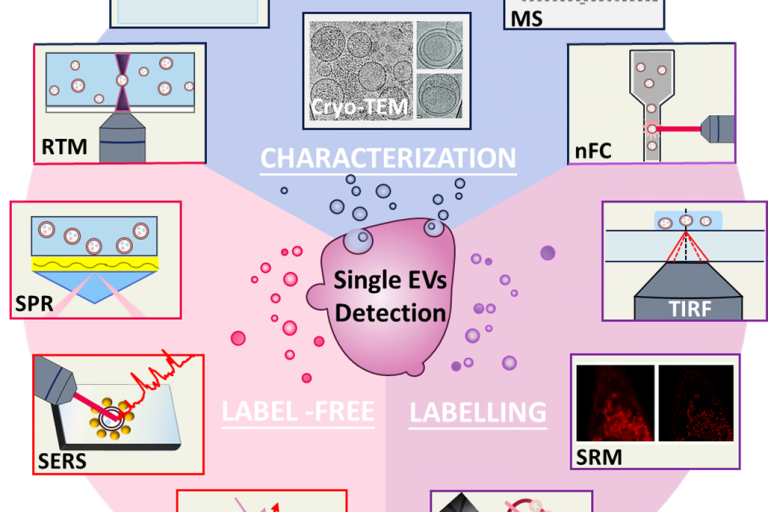
Analysis of Single Extracellular Vesicles for Biomedical Applications with Especial Emphasis on Cancer Investigations
TrAC Trends in Analytical Chemistry Volume 152, July 20…
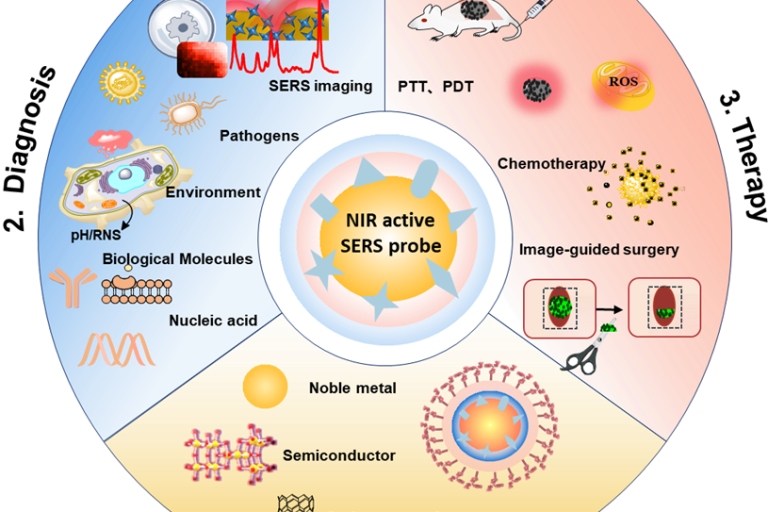
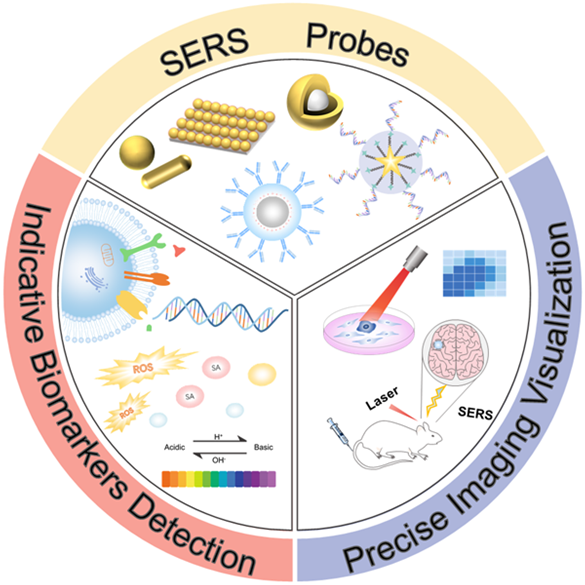
Emergence of Surface-enhanced Raman Scattering (SERS) probes in near-infrared windows for biosensing and bioimaging
Anal. Chem. 2022, 94, 143−164,Full Text Hui Chen, Ziyi …
Toward Sensitive and Reliable Surface-Enhanced Raman Scattering Imaging: From Rational Design to Biomedical Applications
ACS Sens. 2021, 6, 11, 3912–3932,Full text
Shanshan Lin【林珊珊】, Ziyi Cheng【程子译】, Qifu Li【李其富】*, Rui Wang【王锐】*, and Fabiao Yu【于法标】*
Publication Date:November 2, 2021 https://doi.org/10.1021/acssensors.1c01858
Copyright © 2021 American Chemical Society
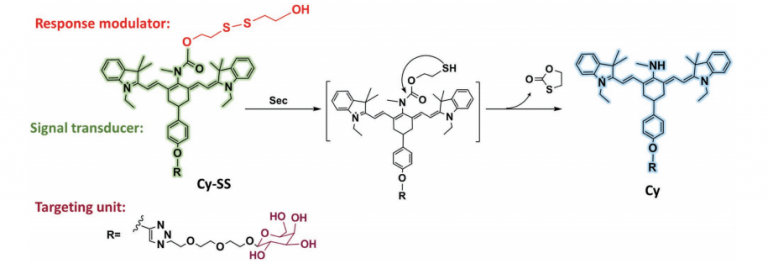
Construction of a biotin-targeting drug delivery system and its near-infrared theranostic fluorescent probe for real-time image-guided therapy of lung cancer
Chinese Chemical Letters . 2021, Full text
Xinyu Song【宋新宇】ad1 Rui Wang【王锐】bc1* Junfang Gaoa Xiaoyue Han【韩潇玥】bc Jianfeng Jin【金剑锋】bc Changjun Lv【吕长俊】a* Fabiao Yu【于法标】bc*
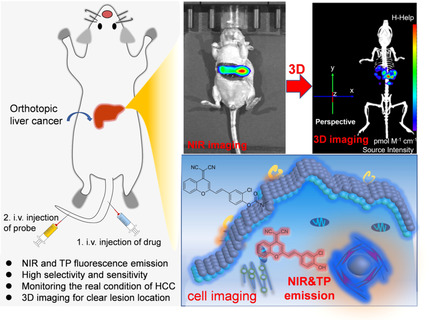
Rational Design of a Highly Selective Near-Infrared Two-Photon Fluorogenic Probe for Imaging Orthotopic Hepatocellular Carcinoma Chemotherapy
Angew. Chem. 2021, 133, 2 – 10Full text
Dr. Xiaofeng Wu, Dr. Rui Wang, Sujie Qi, Dr. Nahyun Kwon, Jingjing Han, Heejeong Kim, Dr. Haidong Li, Prof. Dr. Fabiao Yu*, Prof. Dr. Juyoung Yoon*
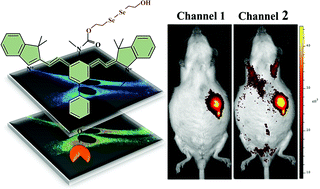
Real-Time Evaluation of Hydrogen Peroxide Injuries in Pulmonary Fibrosis Mice Models with a Mitochondria-Targeted Near-Infrared Fluorescent Probe
ACS Sensor 2021, 6, 3, 1228–1239 Full text
Xinyu Song, Song Bai, Na He, Rui Wang, Yanlong Xing*, Changjun Lv*, and Fabiao Yu*
Indication of Dynamic Peroxynitrite Fluctuations in the Rat Epilepsy Model with a Near-Infrared Two-Photon Fluorescent Probe
Anal. Chem. 2021, 93, 2490−2499 Full text
Xianzhu Luo, Ziyi Cheng, Rui Wang,* and Fabiao Yu*
https://dx.doi.org/10.1021/acs.analchem.0c04529
A semi-naphthorhodafluor-based red-emitting fluorescent probe for tracking of hydrogen polysulfide in living cells and zebrafish
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Volume 247, 15 February 2021, 119105Full text
YingyingMa;ZhencaiXu;QiSun;LinlinWang;HengLiu;FabiaoYu
Tumor Microenvironment-Specific Functional Nanomaterials for Biomedical Applications
Journal of Biomedical Nanotechnology Vol. 16, 1325–1358, 2020Full text
Linlu Zhao, Heng Liu, Yanlong Xing, Rui Wang, Ziyi Cheng, Chuanzhu Lv ∗, Zhiyue Lv∗, and Fabiao Yu∗
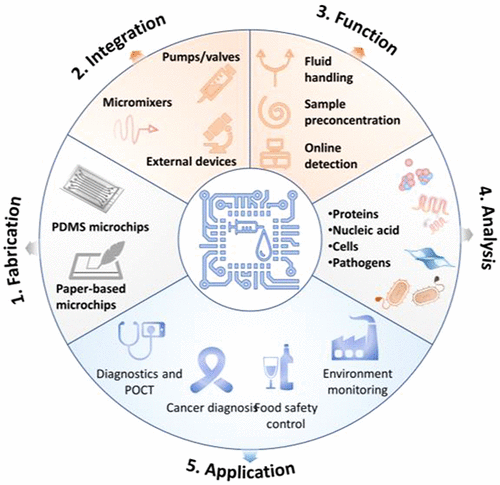
Microfluidics-Based Sensing of Biospecies
Analysis of Extracellular Vesicles as Emerging Theranostic Nanoplatforms.
Coordination Chemistry Reviews. 2020, 424, 213506Full text
Yanlong Xing, Ziyi Cheng, Rui Wang, Chuanzhu Lv,* Tony D. James* and Fabiao Yu*.
Molecular Fluorescent Probes for Imaging and Evaluation of Hypochlorite Fluctuations during Diagnosis and Therapy of Osteoarthritis in Cells and in a Mouse Model
ACS Sensor, 2020, 5,7,1949-1958.Full text
Ji-Ting Hou, Bingya Wang, Yuxia Zou, Peiwen Fan, Xueping Chang, Xinhua Cao*, Shan Wang*, and Fabiao Yu*
https://dx.doi.org/10.1021/acssensors.0c00270
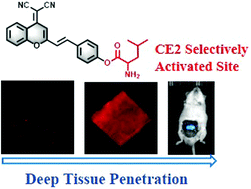
Visualization of carboxylesterase 2 with a near-infrared two-photon fluorescent probe and potential evaluation of its anticancer drug effects in an orthotopic colon carcinoma mice model
Chemical Communications, 2020, 56, 4412 – 4415 Full text
Yan Wang, Feifei Yu, Xianzhu Luo, Mingshun Li, Linlu Zhao* and Fabiao Yu*
https://pubs.rsc.org/en/content/articlelanding/2020/cc/d0cc00297f/
Bioimaging of Glutathione with a Two-Photon Fluorescent Probe and Its Potential Application for Surgery Guide in Laryngeal Cancer
ACS Sensor. 2020, 5, 242−249Full text
Yuxia Zou, Mingshun Li, Yanlong Xing,* Tingting Duan, Xuejun Zhou,* and Fabiao Yu*
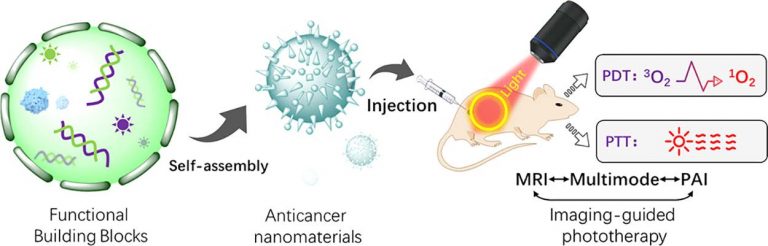
Self-Assembled Nanomaterials for Enhanced Phototherapy of Cancer
ACS Applied Bio Materials. 2020, 3, 86−106Full text
Linlu Zhao, Yanlong Xing, Rui Wang, FeiFei Yu,* and Fabiao Yu*
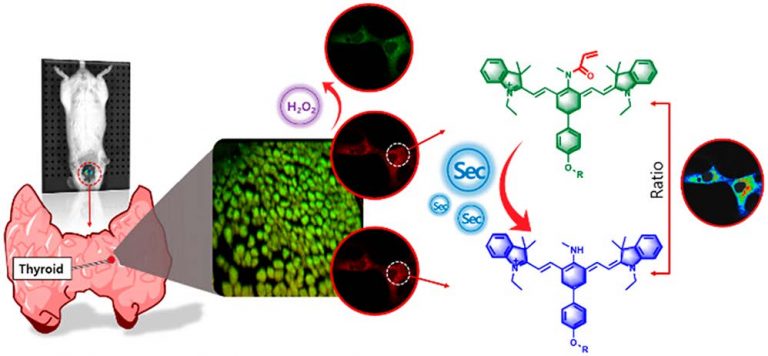
Detection of Selenocysteine with a Ratiometric near-Infrared Fluorescent Probe in Cells and in Mice Thyroid Diseases Model
Analytical Chemistry. 2020, 92, 1589−1597Full text
Xianzhu Luo,Rui Wang, Chuanzhu Lv, Guang Chen,* Jinmao You,* and Fabiao Yu*
Imaging of the mutual regulation between zinc cation and nitrosyl via two-photon fluorescent probes in cells and in vivo
Sensors and Actuators: B. Chemical 309 (2020) 127772Full text
Mingshun Lia, Yanlong Xingb, Yuxia Zoub, Guang Chena,*, Jinmao Youa,*, Fabiao Yua,b,*
https://www.sciencedirect.com/science/article/abs/pii/S0925400520301192
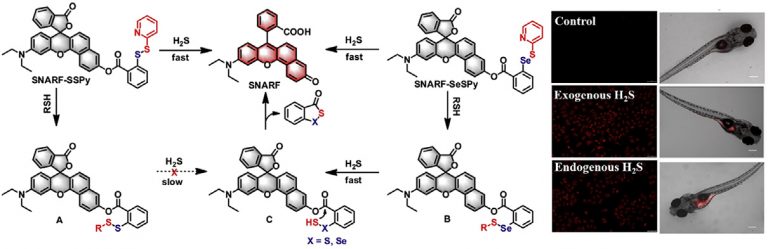
Visualizing hydrogen sulfide in living cells and zebrafish using a redemitting fluorescent probe via selenium-sulfur exchange reaction
Analytica Chimica Acta 1109 (2020) 37e43Full text
Xiaoyu Zhang , Wangbo Qu , Heng Liu , Yingying Ma , Linlin Wang ,Qi Sun ***, Fabiao Yu , *
https://www.sciencedirect.com/science/article/abs/pii/S0003267020302701
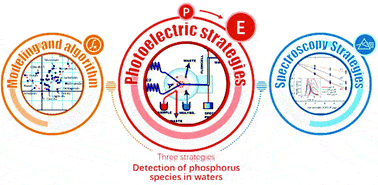
Detection of phosphorus species in water: technology and strategies
Analyst, 2019,144, 7130-7148Full text
Hongwei Chen,Linlu Zhao,Fabiao Yu * and Qiaoling Du*
https://pubs.rsc.org/ko/content/articlelanding/2019/an/c9an01161g/
SERS-based immunoassay using gold-patterned array chips for rapid and sensitive detection of dual cardiac biomarkers
Analyst, 2019, 144, 6533Full text
Ziyi Cheng, Rui Wang, Yanlong Xing, Linlu Zhao,Jaebum Choo *and Fabiao Yu *
https://pubs.rsc.org/en/content/articlelanding/2019/an/c9an01260e/
A sulfydryl-based near-infrared ratiometic fluorescent probe for assessment of acute/chronic mercury exposure via associated determination of superoxide anion and mercury ion in cells and in vivo
Sensors and Actuators B: Chemical, 2019, 127038Full text
Wang, Yue ; Gao, Min ; Liao, Chunyang ; Yu, Fabiao* ; Chen, Lingxin*
https://www.sciencedirect.com/science/article/abs/pii/S0925400519312377
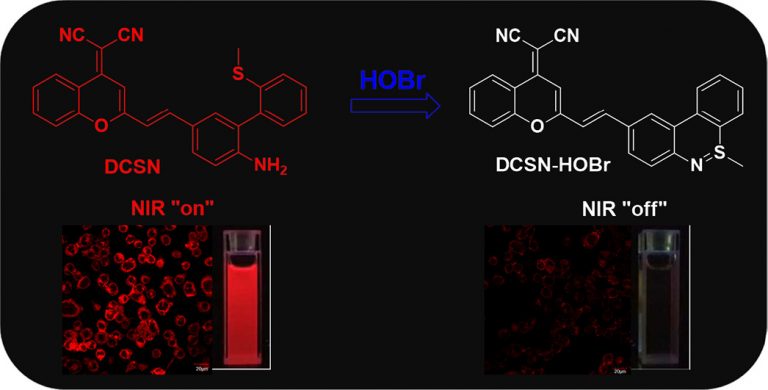
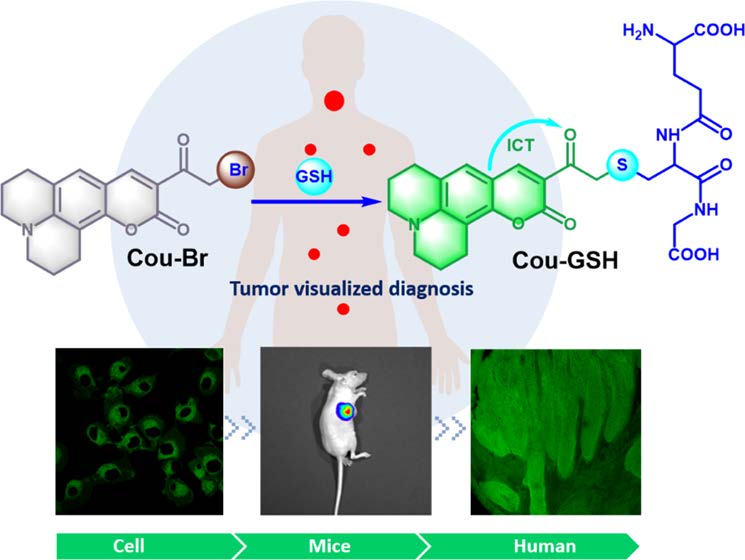
Lysosome-targetable red-emitting ratiometric fluorescent probe for hypobromous acid imaging in living cells
Sensors and Actuators B: Chemical, 2019, 297, 126826Full text
WangboQu,KaibinLi,DemanHan*,XinxinZhong,CaixiaChen,XiuxiaLiang,HengLiu*
https://www.sciencedirect.com/science/article/abs/pii/S0925400519310251
Development of a novel near-infrared fluorescence light-up probe with a large Stokes shift for sensing of cysteine in aqueous solution, living cells and zebrafish
Dyes and Pigments, 2019, 171, 107722Full text
XiaoyuZhang,HengLiu,YingyingMa,WangboQu,HanpingHe,XiuhuaZhang,ShengfuWang,QiSun*,FabiaoYu*
https://www.sciencedirect.com/science/article/abs/pii/S0143720819314500
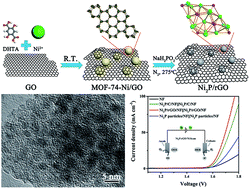
Nickel metal–organic framework implanted on graphene and incubated to be ultrasmall nickel phosphide nanocrystals acts as a highly efficient water splitting electrocatalyst
Journal of Material Chemistry A, 2018, 6, 1682-1691Full text
Liting Yan, Huimin Jiang,Yanlong Xing* ,Ying Wang, Dandan Liu,Xin Gu, Pengcheng Dai, Liangjun Li and Xuebo Zhao *
https://pubs.rsc.org/–/content/articlelanding/2018/ta/c7ta10218f/
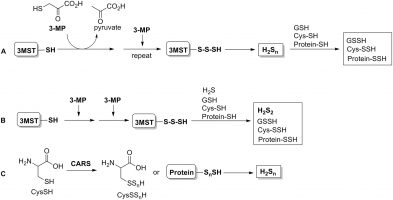
Inorganic hydrogen polysulfides: chemistry, chemical biology and detection.
British Journal of Pharmacology, 2019, 176 (4), 616-627Full text
Heng Liu 1 2, Miles N Radford 2, Chun-Tao Yang 3, Wei Chen 2, Ming Xian 2 3
https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14330
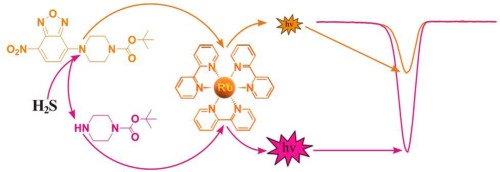
Electrochemiluminescent sensor based on Ru(bpy)32+-doped silica nanoprobe by incorporating a new co-reactant NBD-amine for selective detection of Hydrogen sulfide
Sensors and Actuators B: Chemical, 2019, 284, 451-455Full text
Jing Huang; Wangbo Qu; Junlun Zhu; HengLiu; WeiWen; XiuhuaZhang; ShenfuWang
https://www.sciencedirect.com/science/article/abs/pii/S0925400519300061
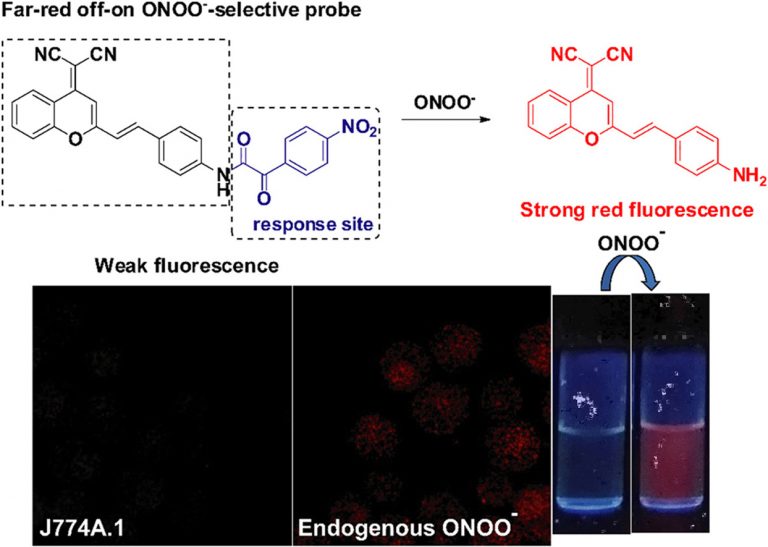
Construction of a novel far-red fluorescence light-up probe for visualizing intracellular peroxynitrite
Talanta, 2019, 197, 431-435Full text
Wangbo Qu 1, Changhe Niu 2, Xiaoyu Zhang 1, Wei Chen 3, Fabiao Yu 4, Heng Liu 5, Xiuhua Zhang 1, Shengfu Wang 1
https://www.sciencedirect.com/science/article/abs/pii/S0039914019300645
A dual response near-infrared fluorescent probe for hydrogen polysulfides and superoxide anion detection in cells and in vivo
Biomaterials, 2015, 63, 93–101Full text
Yu, Fabiao; Gao, Min; Li, Meng; Chen, Lingxin*
https://www.sciencedirect.com/science/article/abs/pii/S0142961215005153
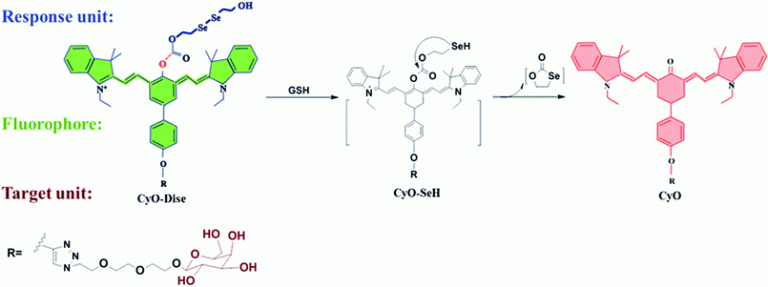
A near-infrared ratiometric fluorescent probe for cysteine detection over glutathione indicating mitochondrial oxidative stress in vivo
Biosensors and Bioelectronics, 2015, 74, 156-164Full text
Kun Yin; Fabiao Yu; Weiwei Zhang; Lingxin Chen
https://www.sciencedirect.com/science/article/abs/pii/S0956566315302074
Wide-Acidity-Range pH Fluorescence Probes for Evaluation of Acidification in Mitochondria and Digestive Tract Mucosa
Analytical Chemistry, 2017, 89, 8509−8516Full text
Chen, Guang ; Fu, Qiang; Yu, Fabiao* ; Ren, Rui; Liu, Yuxia ; Cao, Ziping ;Li, Guoliang;Zhao, Xianen; Chen, Lingxin* ;Wang, Hua; You, Jinmao*
Quantification of cysteine hydropersulfide with a ratiometric near-infrared fluorescent probe based on selenium–sulfur exchange reaction
Chemical Science, 2016, 7, 5098–5107Full text
Han, Xiaoyue; Yu, Fabiao; Song, Xinyu ; Chen, Lingxin*
https://pubs.rsc.org/en/content/articlehtml/2016/sc/c6sc00838k
Near-Infrared Fluorescence Probe for in Situ Detection of Superoxide Anion and Hydrogen Polysulfides in Mitochondrial Oxidative Stress
Analytical Chemistry, 2016, 88,7, 4122-4129Full text
Huang, Yan ; Yu, Fabiao*; Wang, Jianchao; Chen, Lingxin*
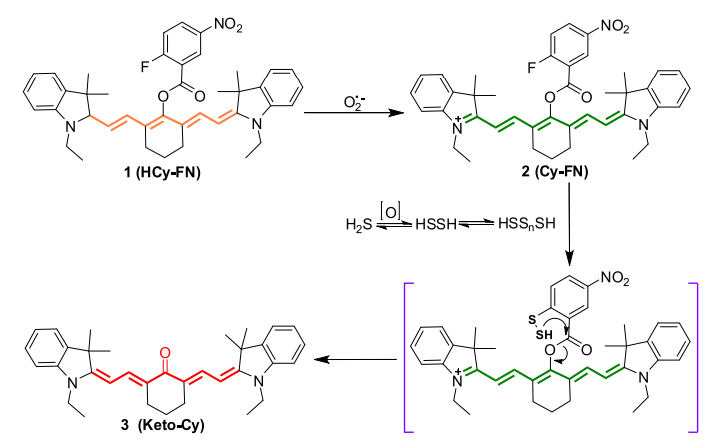
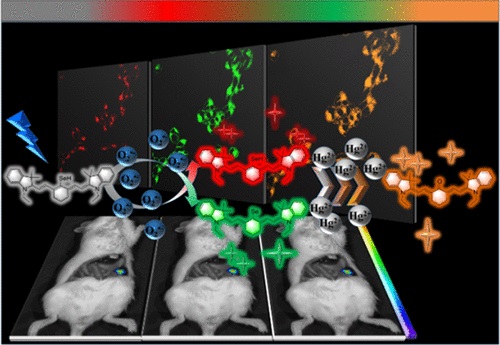
Associated Detection of Superoxide Anion and Mercury(II) under Chronic Mercury Exposure in Cells and Mice Models via a Three-Channel Fluorescent Probe
Analytical Chemistry, 2018, 90, 16, 9769-9778Full text
Wang, Yue;Gao, Min ; Chen, Qingguo ;Yu, Fabiao*; Jiang, Guibin ; Chen, Lingxin*
https://pubs.acs.org/doi/abs/10.1021/acs.analchem.8b01442
Abstract
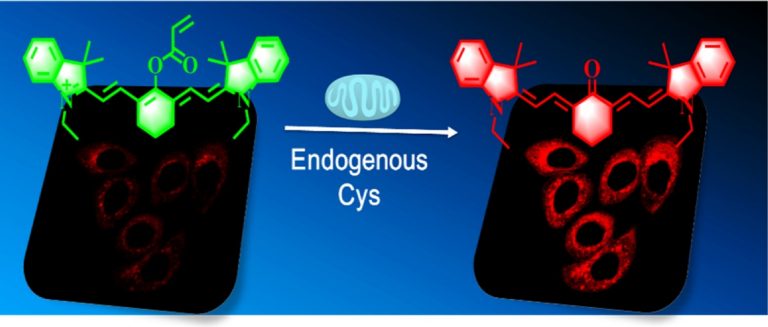
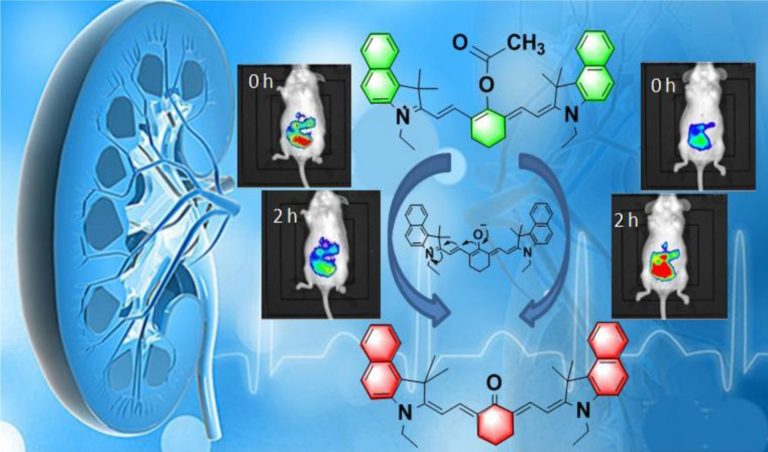
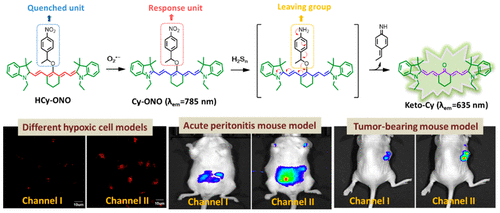
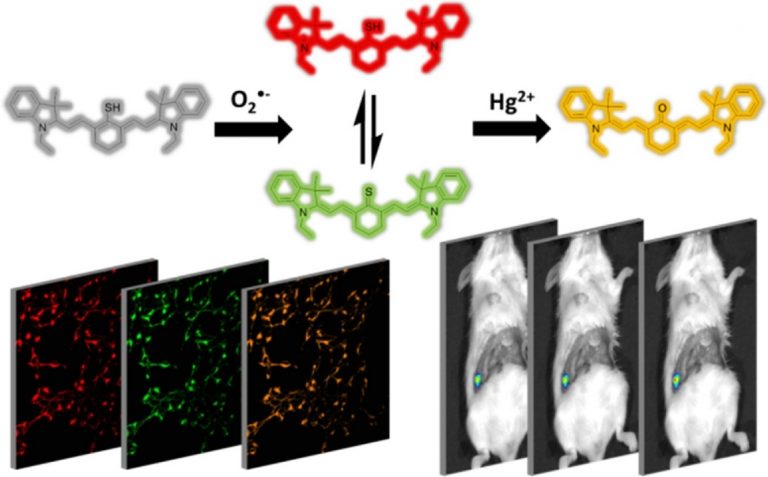
As a cytotoxic heavy metal ion, mercury(II) ion (Hg2+) induces severe oxidative stress and further results in physiological dysfunction. Although mercury poisoning can be treated with many drugs, such as sodium selenite, the therapeutic effect is relatively poor, and it seems that the damage to human health continues. However, the interpretation for the pathogenesis has not been clarified yet. We supposed that the reason is attributed to Hg2+-caused intracellular oxidative stress. To confirm our hypothesis, we strived to design a three-channel ratio fluorescent probe, HCy–SeH, for superoxide anion (O2•–) and Hg2+combined detection. O2•– is a vital precursor for other reactive oxygen species (ROS), which is involved in many physiological and pathological processes. However, until now there is no efficient chemical tool for O2•– and Hg2+ combined detection in cells and in vivo. The fluorescence response of our probe is initiated by a hydrogen abstraction reaction from the hydrocyanine fluorophore moiety. Once oxidized by O2•–, HCy–SeH recovers its π-conjugated system back to a heptamethine cyanine derivative, Cy–SeH. Cy–SeH coexists with its conjugate base, Cy═Se. One emits red fluorescence, and the other one emits green fluorescence. The response unit, −SeH, can trap Hg2+ via a Se–Hg antagonism reaction to afford an orange-emitting final product, Keto–Cy. The probe offers high selectivity and sensitivity toward O2•– and Hg2+. When applied for O2•– and Hg2+detection in HEK 293 cells, the imaging results indicate that our probe can provide a combined response for O2•– and Hg2+ in real time and in situ. Flow cytometry analysis is well-consistent with the results from fluorescence imaging. When applied to image O2•– and Hg2+ in mice models, we find that Hg2+dominantly accumulates in the kidney and induces a burst of O2•–. We confirm that chronic mercurialism can cause severe oxidative damage and renal fibrosis. HCy–SeH further provides a new information that, even when intracellular Hg2+ has been antagonized, the outbreak of O2•– caused by mercury poisoning still lasts.
A ratiometric fluorescent probe for imaging and quantifying anti-apoptotic effects of GSH under temperature stress
Chemical Science, 2017, 8, 6991–7002Full text
Han, Xiaoyue; Song, Xinyu ; Yu, Fabiao*; Chen, Lingxin*
https://pubs.rsc.org/lv/content/articlehtml/2017/sc/c7sc02888a
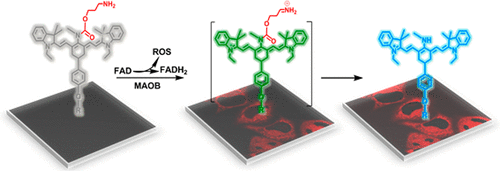
Ratiometric near-infrared fluorescent probe for synergistic detection of monoamine oxidase B and its contribution to oxidative stress in cell and mice aging models
Analytical chemistry, 2018, 90, 4054–4061Full text
Wang Rui ,Han Xiaoyue , You Jinmao ,Yu Fabiao*,Chen Lingxin*
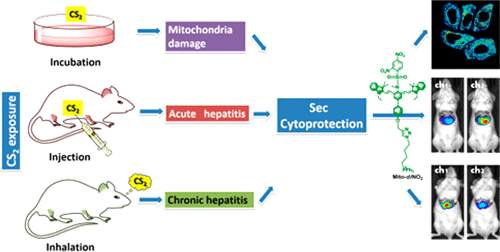
Evaluation Selenocysteine Protective Effect in Carbon Disulfide Induced Hepatitis with a Mitochondrial Targeting Ratiometric Near-Infrared Fluorescent Probe
Analytical chemistry 2018, 90, 8108–8115Full text
Han Xiaoyue ,Wang Rui , Song Xinyu,Yu Fabiao* ,Chen Lingxin*
Imaging of intracellular sulfane sulfur expression changes under hypoxic stress via a seleniumcontaining near-infrared fluorescent probe
Journal of Materials Chemistry B, 2018, 6, 6637-6645Full text
Gao Min, Wang Rui, Yu Fabiao* ,Li Bowei, Chen Lingxin*
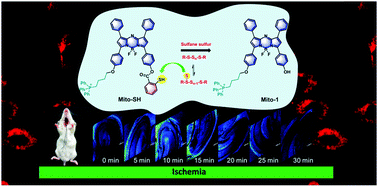
Imaging and evaluation of sulfane sulfur in acute brain ischemia using a mitochondria-targeted near-infrared fluorescent probe
Journal of Materials Chemistry B, 2018, 6, 2608-2619
Gao, Min; Wang, Rui ; Yu, Fabiao*; Youc, Jinmao ; Chen, Lingxin*
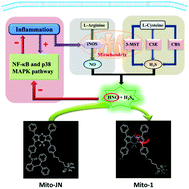
Imaging of anti-inflammatory effects of HNO via a near-infrared fluorescent probe in cells and in rat gouty arthritis model
Journal of Materials Chemistry B, 2019, 7, 305-313Full text
Huang Yan , Zhang Xia ,He Na,Wang Yue, Kang Qi*,Shen Dazhong,Yu Fabiao* ,Chen Lingxin*
Mitochondria-targeting near-infrared ratiometric fluorescent probe for selective imaging of cysteine in orthotopic lung cancer mice
Sensors and Actuators B: Chemical, 2019, 282, 69-77Full text
Zhang Xiaoyu, He Na ,Huang Yan, Yu Fabiao* ,Li Bowei , Lv Changjun *,Chen Lingxin*
A highly sensitive near-infrared ratiometric fluorescent probe for imaging of mitochondrial hydrazine in cells and in mice models
Sensors and Actuators B: Chemical, 2019, 286, 69-77Full text
Song Yaqun, Chen Guang* ,Han Xiaoyue , You Jinmao* , Yu Fabiao*
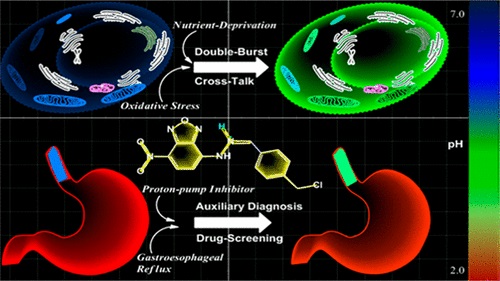
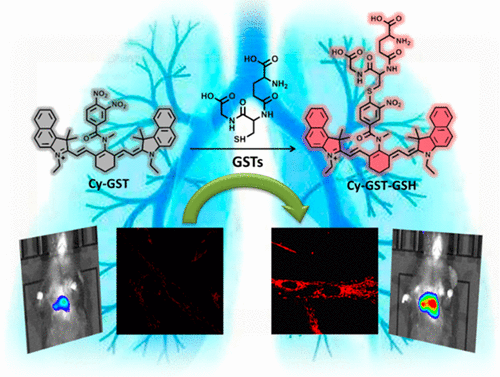
Evaluation of Glutathione S-Transferase Inhibition Effects on Idiopathic Pulmonary Fibrosis Therapy with a Near-Infrared Fluorescent Probe in Cell and Mice Models
Analytical Chemistry, 2019, 91,8,5424-5432Full text
He Na ,Bai Song, Huang Yan , Xing Yanlong , Chen Lingxin, Yu Fabiao , Lv Changjun
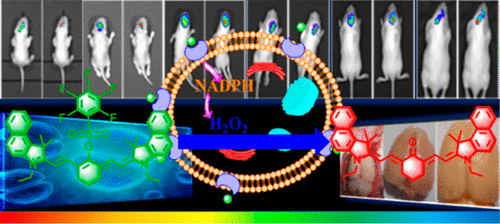
Imaging of Endogenous Hydrogen Peroxide during the Process of Cell Mitosis and Mouse Brain Development with a Near-Infrared Ratiometric Fluorescent Probe
Analytical Chemistry, 2019, 91, 1203-1210Full text
Guo Hailong, Chen Guang, Gao Min , Wang Rui , Liu Yuxia, Yu Fabiao
A Ratiometric Near-Infrared Fluorescent Probe for Quantifcation and Evaluation of Selenocysteine-Protective Effects in Acute Inflammation
Advanced Functional Material, 2017, 27, 1700769Full text
Xiaoyue ,Han Xinyu, Song Fabiao Yu*, Lingxin Chen*
Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy
Chemical Society Review, 2017, 46, 2237-2271Full text
Gao Min, Yu Fabiao , Lv Changjun , Choo Jaebum , Chen Lingxin
A novel near-infrared fluorescent probe for detection of hypobromous acid and its bioimaging applications
Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2019, 222, 117240Full text
Qu Wangbo, Zhang Xiaoyu , Ma Yingying , Yu Fabiao , Liua Heng
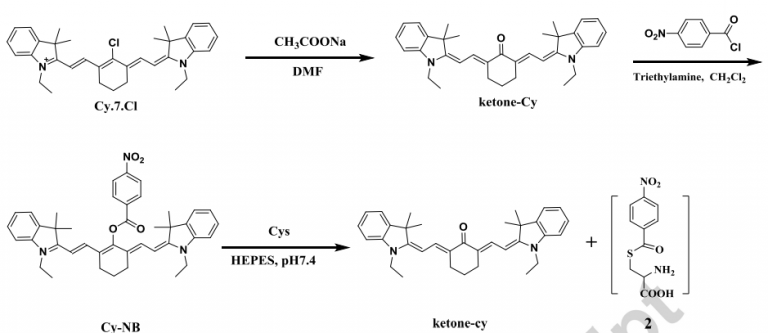
Sequential Detection of Superoxide Anion and Hydrogen Polysulfides under Hypoxic Stress via a Spectral-Response-Separated Fluorescent Probe Functioned with a Nitrobenzene Derivative
Anal. Chem, 2019, 91, 12, 7774-7781Full text
Gao Min , Zhang Xia, Wang Yue,Liu Qingluan ,Yu Fabiao, Huang Yan, Ding Caifeng, Chen Lingxin
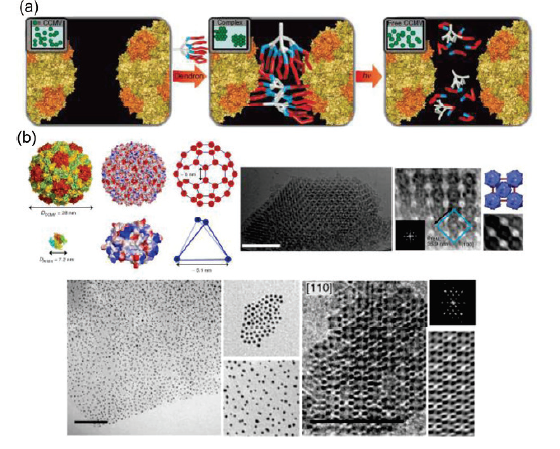
Photocontrolled protein assembly for constructing programmed two-dimensional nanomaterials.
Journal of Material Chemistry B, 2018, 6, 75-83Full text
Zhao Linlu, Li Yijia, Wang Tingting, Qiao Shanpeng , Li Xiumei , Wang Ruidi, Luo Quan , Hou Chunxi , Xu Jiayun ,Liu Junqiu
https://pubs.rsc.org/az/content/articlehtml/2018/tb/c7tb02826a
Development of a novel benzothiadiazole-based fluorescent turn-on probe for highly selective detection of glutathione over cysteine/homocysteine.
Sensors and Actuators B: Chemical, 2018, 266, 528-533Full text
Chen Fengzao , Zhang Jian, Qu Wangbo , Zhong Xinxin , Liu Heng , Ren Jun , He Hanping ,Zhang Xiuhua , Wang Shengfu
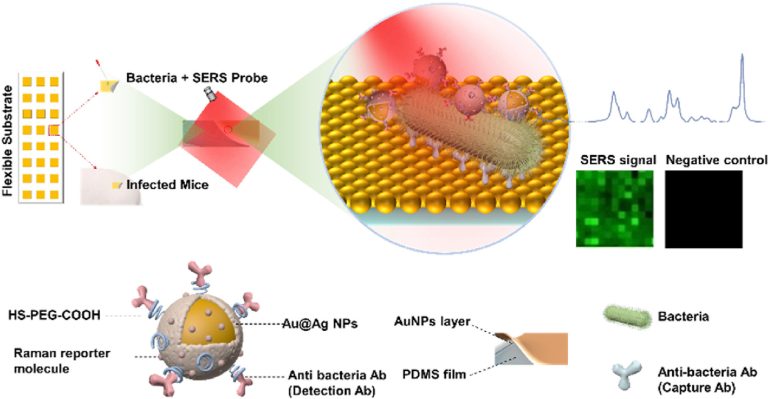
Highly sensitive detection of high-risk bacterial pathogens using SERS-based lateral flow assay strips
Sensors and Actuators B: Chemical, 2018, 142, 91-96Full text
Wang Rui, Kim Kihyun , Choi Namhyun , Wang Xiaokun , Lee Jiyoung, Jeon Jun Ho, Rhie Gi-eun , Choo Jaebum
Imaging of intracellular sulfane sulfur expression changes under hypoxic stress via a selenium-containing near-infrared fluorescent probe
Journal of Materials Chemistry B, 2018, 6, 6637-6645Full text
Gao Min, Wang Rui , yu Fabiao, Li Bowei, Chen Lingxin
https://pubs.rsc.org/–/content/articlelanding/2018/tb/c8tb01794h
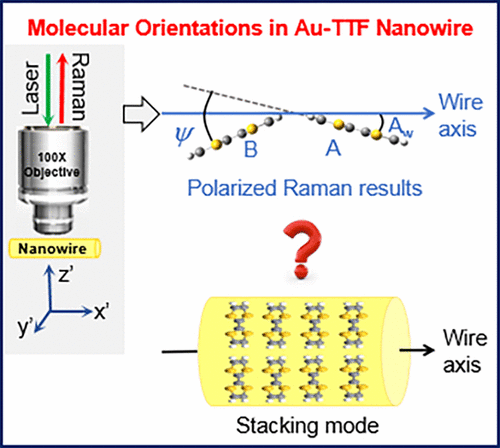
Bi-Axial Growth Mode of Au–TTF Nanowires Induced by Tilted Molecular Column Stacking
Journal of Physical Chemistry C, 2017, 121, 23200–23206Full text
Yanlong Xing, Eugen Speiser, Dheeraj K. Singh, Petra S. Dittrich, and Norbert Esser
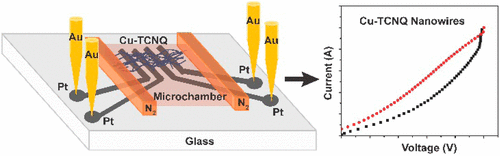
Localized Synthesis of Conductive Copper-Tetracyanoquinodimethane Nanostructures in Ultra-small Microchambers for Nanoelectronics.
ACS Applied Materials and Interfaces, 2017, 9 (20), 17271–17278Full text
Yanlong Xing, Guoguang Sun, Eugen Speiser, Norbert Esser, and Petra S. Dittrich*
Protein self-assembly: technology and strategy
Science China Chemistry, 2016, 59, 1531-1540Full text
Linlu Zhao, Shanpeng Qiao ,Junqiu Liu
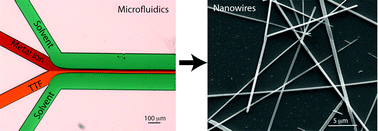
Conductive Single Nanowires Formed and Analysed on Microfluidic Devices
Journal of Material Chemistry C, 2016, 4, 9235–9244Full text
Xing Yanlong,Esser Norbert , Dittrich Petra S.
https://pubs.rsc.org/en/content/articlelanding/2016/tc/c6tc02791a/
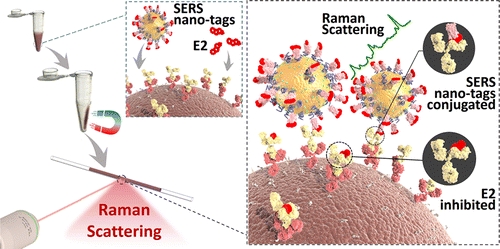
Highly Sensitive Detection of Hormone Estradiol E2 Using Surface-Enhanced Raman Scattering Based Immunoassays for the Clinical Diagnosis of Precocious Puberty
ACS Applied Materials & Interfaces, 2016, 8, 10665-10672Full text
Wang Rui ,Chon Hyangah,Lee Sangyeop , Cheng Ziyi, Hong Sung Hyun, Yoon Young Ho, Choo Jaebum
Label-free biosensors based on in situ formed and functionalized microwires in microfluidic devices
Analyst, 2015,140, 7896–7901Full text
Xing Yanlong , Wyss Andreas, Esser Norbert,Dittrich Petra S.
https://pubs.rsc.org/en/content/articlelanding/2015/an/c5an01240f/


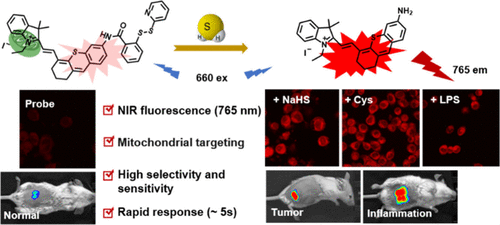
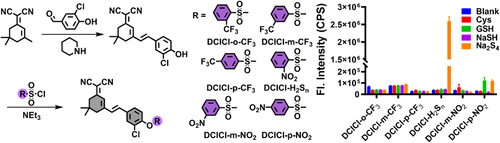
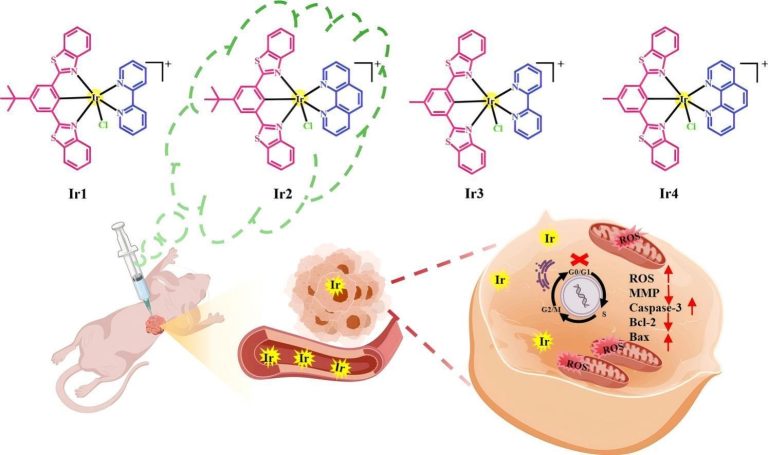
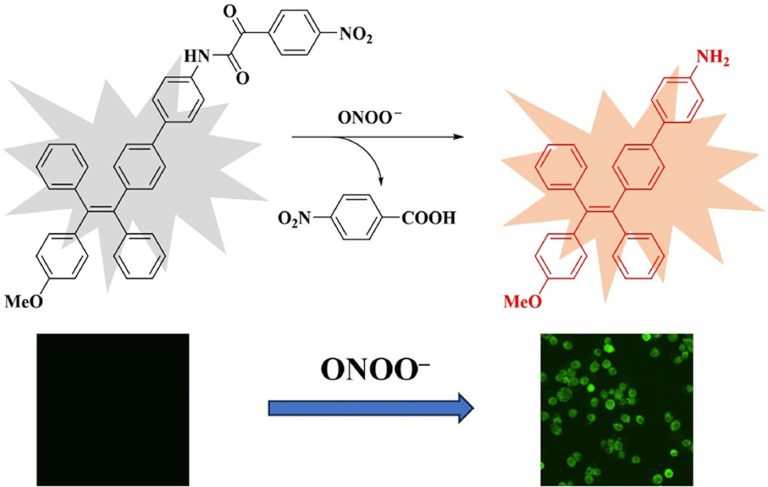
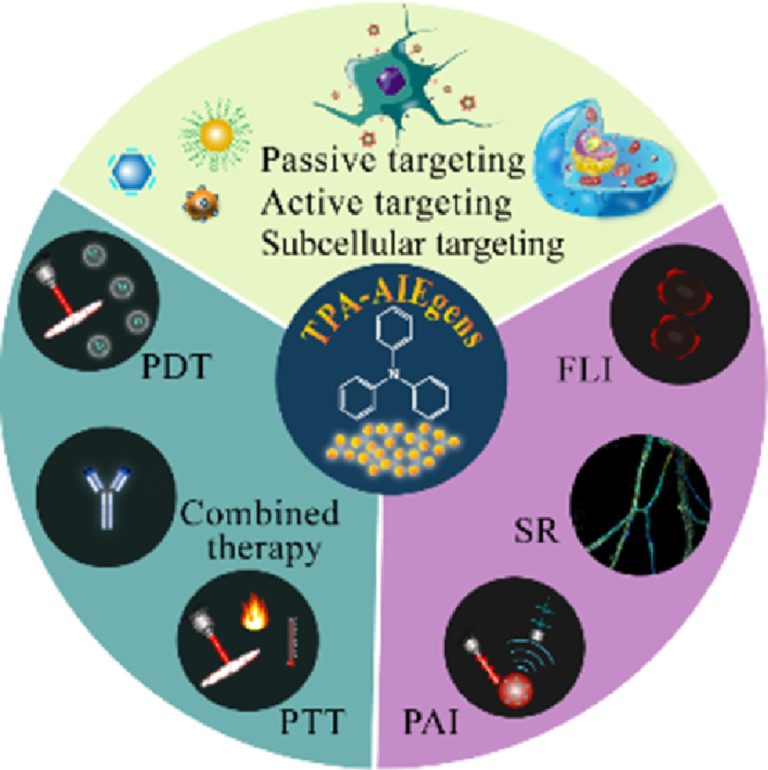
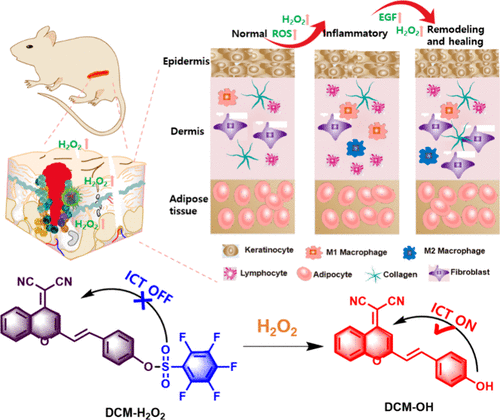
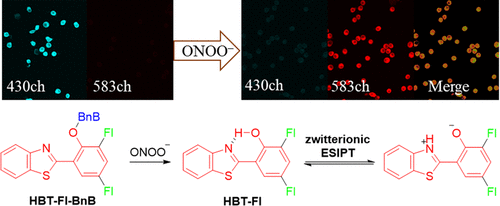
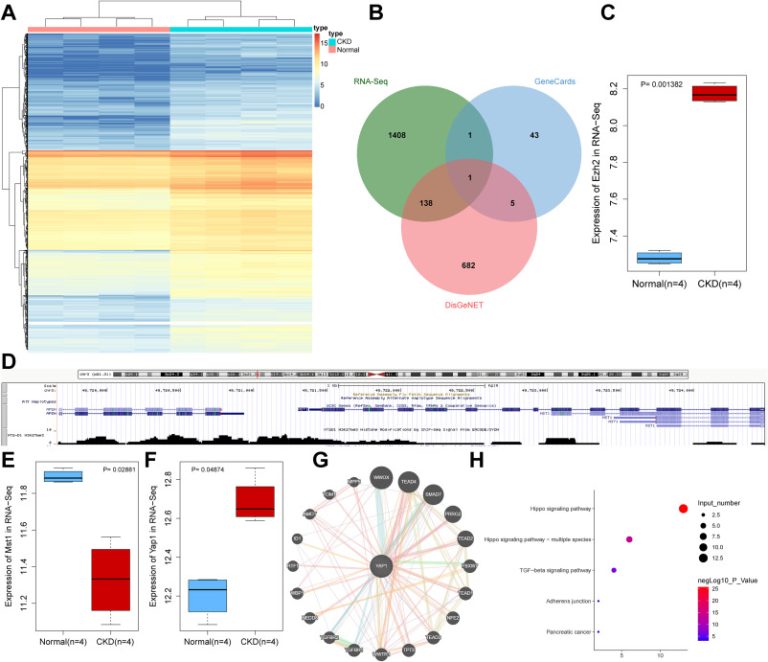
![1594603261-se0c00270_0008[1]](https://www.ifmmi.com/wp-content/uploads/2020/07/1594603261-se0c00270_00081.gif)

![1-s2.0-S0925400519310251-ga1_lrg[1]](https://www.ifmmi.com/wp-content/uploads/2019/07/1-s2.0-S0925400519310251-ga1_lrg1-768x364.jpg)